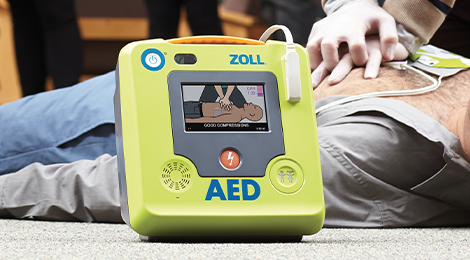
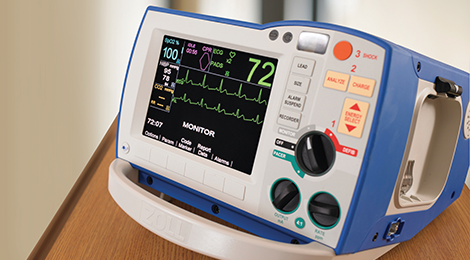
Public Access Defibrillation
Designed with lay rescuers in mind, ZOLL® AEDs combine advanced CPR feedback technology, ease of use, and reliability to support you throughout the rescue. Research shows that real-time CPR feedback, along with training, more than doubles cardiac arrest survival rates.1 All of our AEDs support AHA and ERC guidelines for high-quality CPR. Choose a best-in-class AED from ZOLL and have confidence that you are ready for the rescue.
ZOLL AED 3
The ZOLL AED 3® defibrillator features real-time CPR feedback, full-color rescue images, RapidShock™ analysis technology, and an integrated child mode, making it simple to treat both adult and pediatric victims of SCA. Designed with readiness in mind, ZOLL AED 3 includes long-life consumables and optional WiFi connectivity for remote monitoring of the AED's status.
Powerheart G5
The Powerheart® G5 AED with Intellisense™ CPR coaches rescuers with real-time corrective feedback on rate, depth, and recoil to ensure delivery of quality CPR compressions. Perform each step of the rescue with user-paced instruction from RescueCoach® voice and text prompts. Fully automatic shock delivery, one-button dual-language functionality and a daily self-test give rescuers the power to act with confidence.
AED Plus
The ZOLL AED Plus® defibrillator with Real CPR Help® technology helps rescuers provide high-quality CPR and will deliver a shock if needed. Real-time CPR feedback on compression rate and depth gives lay rescuers confidence and clarity throughout the rescue.
Program Management
Your AED needs to be ready the day you need it, not just the day you buy it. PlusTrac™ AED program management software helps you track and manage consumable items such as pads and batteries, monitors the certification expiration dates of volunteer responders, and keeps track of your compliance with local AED regulations.
Professional Defibrillation
ZOLL provides AEDs and defibrillators for trained medical professionals, dental offices, urgent care centres, nursing homes, and other out-of-hospital care facilities. Our clinically advanced, user-friendly products help trained professionals respond quickly and effectively when sudden cardiac arrest occurs. Choose from our diverse line of professional products.
ZOLL AED 3 BLS
Designed for professional rescuers, the ZOLL AED 3® BLS automated external defibrillator provides in-depth rescue support for both adult and pediatric victims of sudden cardiac arrest and is one of the fastest AEDs in the industry at delivering a shock after chest compressions stop. Optional WiFi connectivity allows the AED to report readiness status and to transmit event data. It also features a CPR Dashboard, which shows elapsed time, CPR cycle countdown, shocks delivered, and ECG.
AED Pro
The AED Pro® defibrillator is designed to perform in any environment and can be used by professional or lay rescuers. Proprietary Real CPR Help and See-Thru CPR® technologies provide the advanced functionality that rescuers need to perform optimal CPR and increase an SCA victim’s chance of survival.
R Series
The R Series® monitor/defibrillator is the worldwide choice of hospitals for in-patient resuscitation. It features a full suite of tools to support CPR, including a complete CPR Dashboard for both adults and pediatrics, See-Thru CPR filtering, and OneStep™ adult and pediatric electrodes that enable Real CPR Help to provide real-time visual feedback on compression rate and depth.
1Bobrow BJ, et al. Ann Emerg Med. 2013 Jul;62(1):47–56.e1. Epub 2013 Mar 7.